At first glance, this may seem like a silly question, but there are no silly questions in life and in fact, “does metal burn in a fire?” is a complicated question with quite a few possible answers. So, we’ve put together a guide to walk you through the answers.
Metals can burn or melt in a fire, depending on the temperature and type of metal. Some metals such as sodium and magnesium will burn, even explosively, in the presence of oxygen and a flame.
There are many different kinds of metal and this is what you need to know about how and why they burn. Let’s take a closer look at metals and how they react to fire. We will also take a look at some of the specific types of metals that react differently in a fire. Check it out.
Your # 1 priority is keeping your family safe. As a firefighter, I recommend everyone has updated smoke detectors that don’t require battery changes, like these ones from Kidde, a fire extinguisher, like this one from Amerex, and a fire escape ladder if you have bedrooms above the first floor, I recommend this one from Hausse.
Also read: How Do You Put Out A Magnesium Fire?
What Is A Fire?
Before we look at how metals burn, it’s a good idea to remind ourselves what a fire is.
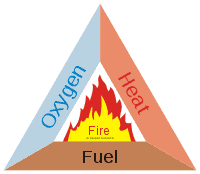
Basically, all fires have three components which are the “fire triangle”, if you like. They are:
- Heat – a fire won’t burn at absolute zero, because it requires an exothermic transfer of energy (usually by consuming the other two components of the fire) and that means there will be heat and usually a flame present too.
- Fuel – it doesn’t matter what kind of fire it is, it can’t burn without having something to burn. In the engine of your car, gasoline is burned to release heat, which is used to drive the motion of the engine and thus the vehicle as a whole. If you make a bonfire in the autumn, leaves, and wood will be the fuel. And so on.
- Oxygen – fuel doesn’t burn by itself, it requires the presence of an oxidizer and in the vast majority of fires, that means oxygen (there are chemical reactions which use other forms of oxidizer but you are unlikely to encounter these outside of specific laboratory techniques) which binds with the fuel in the chemical reaction of the fire.
Also read: Is Copper Flammable?
Is Metal Flammable?

The majority of metals are not flammable. They have ignition points so high that you might need a nuclear furnace for them to catch fire. That doesn’t mean they won’t melt, just that they won’t sustain a fire by themselves.
However, there are some flammable metals and these are classified by the simple idea that if the material is on fire and you remove the heat source, it will continue to burn.
It is worth noting that while these metals are typically highly reactive, this is not always the case.
Aluminum, for example, is not flammable in solid form, when present in large chunks, but if you make a powder out of it? Then it’s flammable. The resulting product, Aluminum Oxide is, thankfully, non-toxic and thus will not create a hazard but the fire? That will burn very hot.
The big danger of working with a powder is not so much that you will accidentally catch it on fire from a match or lighter, but that it may catch fire due to an electrical discharge, which ignites the powder when it’s hanging in a cloud over the workspace.
More worryingly, if this fire comes into contact with water, it will release hydrogen gas and if there’s one thing even more flammable than aluminum powder, it’s raw hydrogen. It will form water again but only after it’s exploded and burned a lot of things around it.
Other flammable metals include magnesium and titanium. The most common metal fires are made up of these two metals.
Also read: Is Sand Flammable? What You Should Know
What Are Combustible Metals?
The combustible metals are those which are easily burned at around room temperature with only a mild heat source applied to them. These include sodium, lithium, potassium, calcium, uranium, cesium, and plutonium.
Fortunately, the vast majority of people won’t be spending too much time around these chemicals. Because of their highly reactive nature, they tend to be used in chemistry laboratories, but are not found around the home. The compounds of metals don’t have the same properties as the metal they derive from.
For example, Sodium Chloride, is made from Sodium (a highly reactive metal) and Chlorine (a very corrosive and dangerous gas) but put them together? You’ve got table salt which offers no real threat to anyone unless you have high blood pressure or a thyroid problem.
Also read: Is Aluminum Flammable?, Is Lead Flammable?, Is Iron Flammable?, and Is Magnesium Flammable?
What Is A Class D Fire?
Metal fires are known as class D fires and they tend to only occur in labs, factories, warehouses, etc., where these metals are found.
Take a look at this magnesium fire demonstration:
They can be extinguished by the use of a Class D fire extinguisher which is a dry powder extinguisher.
The idea is to spray the powder so that it completely coats the fire. This will cut the oxygen off from the metal and extinguish the flames.
The powder prevents you from blowing burning metal or metal powder around while you extinguish it. This can significantly reduce the impact of the fire on the environment it is in.
Never reach for a water fire extinguisher to put out a metal fire.
The temperatures may reach up to 5,000 degrees Fahrenheit. This means when the water hits the flame, it may break down into its constituent elements – hydrogen and oxygen. We touched on this with the aluminum powder example, but if you release the hydrogen gas into your environment – the fire is likely to turn explosive.
However, it is worth noting that as with all things fire-related, it is far better to prevent a fire from starting in the first place than to try and put one out later.
The good news is that the use of these metals is pretty strictly regulated, and most workplaces are aware of the fire safety checks and storage requirements for them.
If you work somewhere that doesn’t regularly practice drills, have working emergency lighting installed and dry powder fire extinguishers on hand and you’re working with combustible metals – then you should raise your concerns with management immediately.
Class D metal fires are no joke and because of the extreme heat that they produce, they can often be far more dangerous than other fires.
Also read: Is Sodium Flammable?
Does Metal Burn In Fire?
Yes, and the easiest example if you want to see this in action is in the use of plasma cutting equipment or oxy-acetylene torches.
Here, the metal is quite literally burned in order to cut through the surface in a straight line. It happens at very high temperatures and the resulting compounds form a gas that can be easily blown out of the space that the cut leaves behind so that it doesn’t interfere with the cutting.
If you’ve not seen this kind of cutting before, you can watch it in action here:
Iron can also be burned easily if it is reduced to a very fine powder in “thermite” reactions. This is used to do the opposite of cutting and is often used for welding purposes.
You can see a demonstration of the thermite reaction below:
In most cases, steel and iron in ordinary buildings will not burn during a fire, because the temperatures of the fire don’t get high enough for them to reach ignition temperature, but this doesn’t mean that given the right circumstances that they can’t burn.
Also read: Is Graphite Flammable? Explained
Conclusion
Does metal burn in a fire? Yes, in fact, one of the hottest reactions in the world is the use of a thermite grenade (which can be used to weld together train tracks, for example) and in this reaction, you set fire to aluminum. The aluminum binds with the oxygen in the air to form an aluminum oxide which has very different properties from aluminum.
However, in order to get aluminum to burn in the first place, you need the thermite to generate the heat – many metals won’t burn without extremely high temperatures. On the other hand, metals such as Sodium, Potassium, Magnesium, etc. are much more reactive and can be very easily burned using nothing but a naked flame. Others such as Cesium are so reactive that they will explode if they come into contact with the air!
Related Articles:
Is Gold Flammable? Surprising Answer…

