Hydrogen Peroxide is a chemical that is quite common for many uses. Like most chemicals, it is important to understand how it behaves, so that you can be aware of any risks or hazards while using it. People commonly ask whether or not Hydrogen Peroxide is a flammable chemical and whether or not it will burn when exposed to flame or other ignition sources.
Hydrogen peroxide is not a flammable substance, however, it can cause a fire to intensify in some cases. Though it will not technically fuel a fire, it can be a fire hazard when combined with other substances.
To understand the fire hazards associated with hydrogen peroxide, we need to take a look at the elements necessary for fire. We will also look at the specific hazards of hydrogen peroxide. Check it out.
Your # 1 priority is keeping your family safe. As a firefighter, I recommend everyone has updated smoke detectors that don’t require battery changes, like these ones from Kidde, a fire extinguisher, like this one from Amerex, and a fire escape ladder if you have bedrooms above the first floor, I recommend this one from Hausse.
Also read: What Makes Something Flammable?
Hydrogen Peroxide (H2O2)

Hydrogen peroxide can be used for everything from cleaning your bathroom, to getting stains out of clothes, to getting rid of mold or algae, to whitening your teeth.
While we see this chemical used all the time, it can be dangerous if not used properly.
Hydrogen peroxide is not classified as a flammable or combustible liquid as it will not fuel fire on its own. While it will not act as a fuel to a fire, it can make a fire more intense.
To understand how hydrogen peroxide behaves in a fire, let’s take a look at the elements that make a fire.
All fire needs 3 things: Heat, Fuel, and Oxygen. These are represented by the fire triangle.
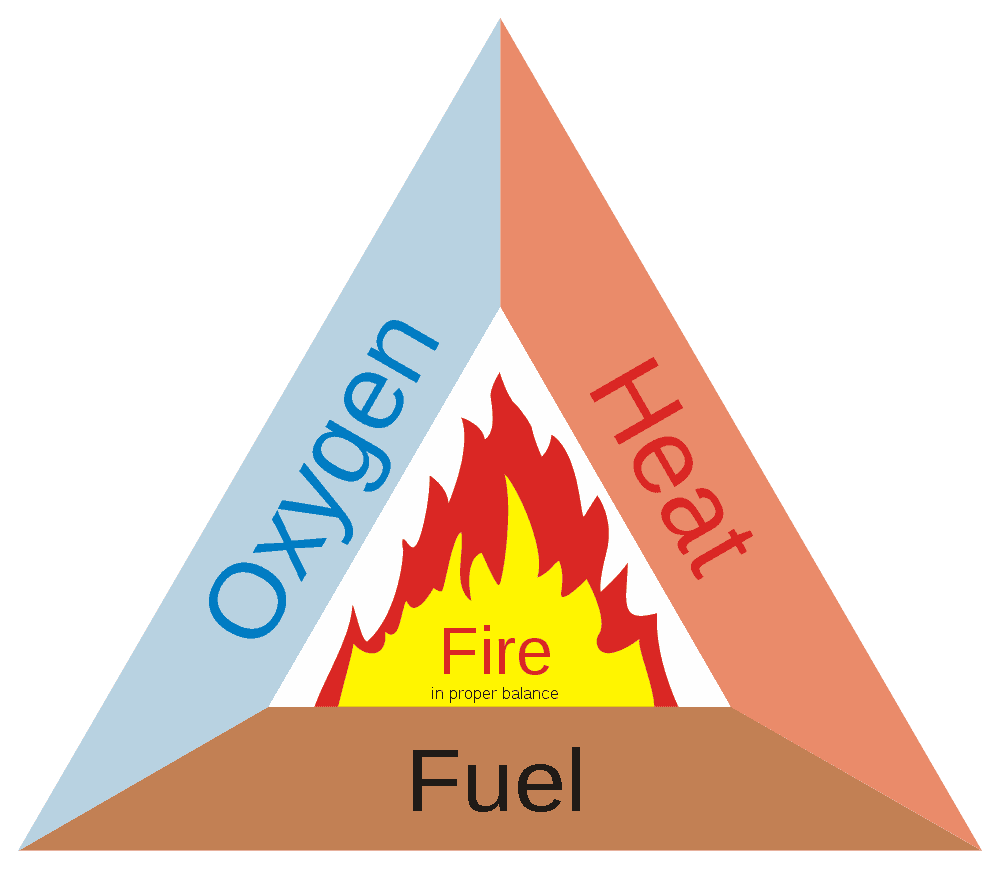
In order for a fire to start and to continue burning, it needs all three of these things in the right amounts. Remove any one of these elements and the fire will go out.
This is basically how fires are fought and extinguished. Put enough water on a fire and it will cool it enough to go out. Put sand on a campfire and you separate the fuel from the oxygen and the fire goes out. If a fire in an enclosed space uses all the oxygen, it will put itself out.
Back to the hydrogen peroxide.
Hydrogen Peroxide (or H2O2) is not flammable in that it will not burn as a fuel. However, it is an oxidizer.
An oxidizer is a chemical that adds oxygen or other elements (fluorine or chlorine) that behave the same way. This means that a fire can burn without oxygen if there is an oxidizer present. It also means that oxidizers can greatly intensify fire and even cause an explosion.
The oxygen in the Hydrogen Peroxide can increase the severity of a fire that is burning or make a fire more likely to ignite if there is adequate fuel and heat.
Also read: Is Chlorine Bleach Flammable or Explosive? and Is Nitrous Oxide Flammable? Yes and No…
Is It Explosive?
Depending on the concentration of the hydrogen peroxide, its reaction can be very different.
According to the Code of Federal Regulations, Title 29, Hydrogen Peroxide hazards will vary by concentration:
| H2O2 Concentration | Uses | Hazards |
|---|---|---|
| Less than 8% | Contact lens sterilizer (2%) Over-the-counter Hydrogen Peroxide (3%) Hair bleach (7.5%) | Not hazardous |
| 8 to 28% | Swimming pool shock treatment (27%) | Slightly increased burning rate |
| 28.1 to 52% | Industrial strength Hydrogen Peroxide | Moderate increase in burning rate |
| 52.1 to 91% | Specialty Chemicals | Severe increase in burning rate, capable of detonation or explosive reaction |
| Greater then 91% | Rocket Propellant | Severe increase in burning rate, can cause spontaneous (without ignition source) ignition and explosive reactions |
In high concentrations, hydrogen peroxide can indeed have explosive reactions.
This video shows medium concentration hydrogen peroxide (35%) causing spontaneous ignition:
In this video, high concentration hydrogen peroxide (91%) creates some very interesting reactions:
Also read: Is Baking Soda Flammable? What About Baking Powder?
Conclusion
As we can see, hydrogen peroxide is not flammable or combustible by the technical definition. However, it certainly is a fire hazard, at least in higher concentrations.
Related Articles
At What Temperature Does Cotton Burn? Is it Flammable?
Is Motor Oil Flammable? You May Be Surprised
Is Diesel Flammable? Yes and No…

